Animal Studies for Pharmaceuticals

When companies like Bayer are developing new drugs, animal studies are both scientifically necessary and legally required. The innovative drug products that result from animal research help to improve the lives of patients all over the world.
Treatment for Bone Metastases
About Metastatic Bone Disease
 Bone metastases derive from tumor cells that migrate from the original primary tumor and invade the bone. Here, they form satellite tumors causing severe pain and often morbidity1. Bones are the third most common target organ of metastasis2. Especially tumor cells deriving from prostate and breast cancer are able to form bone metastases3.
Bone metastases derive from tumor cells that migrate from the original primary tumor and invade the bone. Here, they form satellite tumors causing severe pain and often morbidity1. Bones are the third most common target organ of metastasis2. Especially tumor cells deriving from prostate and breast cancer are able to form bone metastases3.
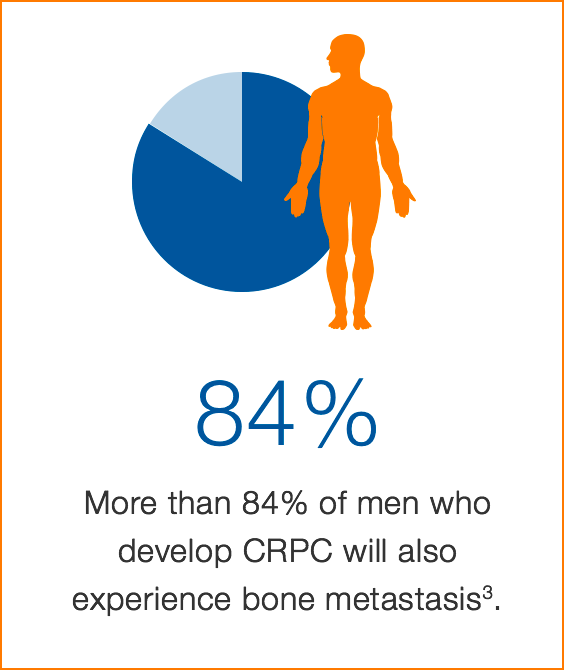
Prostate cancer is the second most common cancer in men worldwide, responsible for over 250,0004 deaths in 2010. Castration resistant prostate cancer (CRPC) is characterized by tumor growth that resumes despite hormone therapy. More than 84% of men who develop CRPC will also experience bone metastasis.
Breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012. Although the number of deaths related to breast cancer has declined over the last years, metastatic cases are still universally incurable5. In about 5% of women metastases are already found at the time point of diagnosis, of which 70% harbor bone metastases6.
What role did animal studies play?
Bayer´s alpha-particle emitting radioactive therapeutic agent was approved in 2013 for the treatment of CRPC patients suffering from bone metastases. In order to test, whether bone metastases originating from breast cancer can be treated by this compound, researchers from Bayer and partners used rodents – mainly mice – to study the anti-tumor activity of the agent in a model of metastatic breast cancer. Each study consisted of a control group of animals which were not treated with the compound, and treatment groups, which received varying doses of the agent. In the study described here, seven mice were in each group. This number was defined by statistical methods to guarantee that the end results would be meaningful.
Once the groups were determined, isolated tumor cells were implanted in the mice to create bone metastases. After the implantation, researchers paid close attention to the mice for several weeks in order to observe the course of the disease, their tolerance of the compound, and their general well-being.
When the study ended at a predefined point, all mice used in the study were euthanized. This is legally required. Samples were taken from each animal to answer questions about the agent’s effects on the tumor and any side effects in healthy organs. Clinical tests in humans began only once the drug was proven to be acceptably safe at the effective dose.
Outcome and Outlook
The animal study described here indicated that the alpha particle-emitting radioactive therapeutic agent can reach and limit the growth of breast cancer bone metastases. It also markedly improved the well-being of the treated mice as compared to untreated animals.
Treatment for Thrombosis
About thrombosis
Thrombosis is a serious and often life-threatening complication of cardiovascular disease in which blood coagulation and platelets are over-activated, forming blood clots that block circulation. Bayer's anticoagulant has been tested in the prevention and treatment of thrombotic and thromboembolic events. It is now approved in more than 130 countries for a wide range of venous and arterial thromboembolism-related indications.
What role did animal studies play?
 The team of scientists who developed the anticoagulant wanted to create an antithrombotic drug that could be taken orally. However, the most effective substance could not be absorbed via the guts of experimental animals. Chemical optimization proved that it was possible to modify the substance to allow absorption from the gut. Animal models were among the tests used to optimize the substance.
The team of scientists who developed the anticoagulant wanted to create an antithrombotic drug that could be taken orally. However, the most effective substance could not be absorbed via the guts of experimental animals. Chemical optimization proved that it was possible to modify the substance to allow absorption from the gut. Animal models were among the tests used to optimize the substance.
During the animal study, scientists induced arterial thrombosis and venous thrombi in anesthetized mice and rats. The selection of the most appropriate animal species was based on in vitro studies comparing the clotting effects in human and animal blood. To reduce the number of animals used in the studies, researchers developed an intravital laser microscope technique that allowed them to use a blood vessel from one animal as both treatment and control. This procedure helped to reduce the number of animals needed in the studies by more than 50 percent.
Outcome and Outlook
The anticoagulant reduced the weight of both the arterial thromboses and the venous thrombi. In 2008, it was approved in the EU for the prevention of venous thromboembolism (VTE) in adult patients following elective knee or hip replacement surgery. Since then, the treatment for thrombosis has been approved for many other indications and is now being tested for other cardiovascular diseases, such as prevention of major adverse cardiac events.








