Bringing Crop Protection Products to the Market
Find out about the regulatory rigor, which ensures that crop protection products put on the market are proven to be safe if applied according to the use instructions.
 | Step 1 Before we are allowed to sell plant protection products, they need to undergo strict evaluation by authorities for each country to ensure that they are safe for humans and have no unacceptable effects on the environment. |
 | Step 2 For this purpose, we need to submit hundreds of residue, toxicological, eco-toxicological, environmental, physical-chemical, and efficacy studies. All this scientific information is compiled in a registration dossier, containing individual study reports, summaries and risk assessments, including proposals for risk mitigation. |
 | Step 3 These dossiers are submitted to regulatory authorities around the world who review the submitted data and risk assessments, and grant or deny approval of active substances and products based on their specific regulations and conclusions. |
This is important to us:
We are not only scientists but also consumers just like you. We take our responsibility for comprehensive, accurate and high-quality safety studies very seriously.
 | To identify one potentially marketable chemical, an agrochemical company screens an average of 160,000 molecules. |
 | Pests, fungi, and weeds develop resistance, this is one reason why there is a constant need for new innovative crop protection products and proper stewardship of the product. |
 | In addition to legally required studies, we perform and submit further studies that go beyond the requirements to thoroughly understand our molecules and prove the compatibility of our products with the environment. We are also obligated to observe additional requirements, such as record keeping, product integrity and informing various regulatory agencies should new information become available. |
When and why do we perform these studies?
Study reports have to be submitted in dossiers to register new active substances and their formulations. The same applies to the renewal of already registered substances and their formulations in many countries. For example, in Europe substance authorizations expire after a maximum of 15 years, which triggers a new application according to latest rules and scientific standards. In the U.S. the authorities regularly review substance authorizations and request new data to reflect regulatory and scientific developments.
The registration process of crop protection products has to follow country- or region-specific guidelines.
The design, performance, and reporting of the studies follow internationally agreed guidance developed by organizations/authorities such as e.g. OECD, EPA, CODEX, EFSA, and others. In general, safety studies are conducted according to international scientific standards, known as Good Laboratory Practice (GLP), that ensure data quality, integrity, and traceability.
Which people in Bayer are involved in the registration process and what are their challenges?

Audit of internal and external laboratories to ensure quality.
Preserve the study records and raw data and comply with audit requests by governmental authorities.
Ensure compliance with documentation, movements, permits, permissions, or any other local, national or international obligations.

They perform these studies in many countries around the globe, analyze the data, perform the risk assessments and write the dossiers.
300-700 safety studies are conducted for authorization of one product, depending on how the product will be used (e.g. on how many crops it can be used).
There can be as many as 80,000 pages per dossier, several hundred pages or more per study report, and even more pages of documented raw data (which are stored for at least 30 years).
Weather conditions have a large impact on outdoor studies that show how products work in the field.

They are the contacts for the regulatory authorities around the globe and they are responsible for preparing all required documents in time.
Some studies are run in parallel around the world and this has to be coordinated.
The test substances used in the studies have to be synthesized in time including all metabolites and other chemical breakdown products which are also assessed by authorities.
Dossiers must be prepared for submission in different countries according to the needs of each regulatory agency.
Authorities look at the dossiers very thoroughly. Therefore, the decision timelines at the authority level can take up to 7 years.
Sometimes more than one company will invest in developing a new chemical. Therefore, each company has rights to the data and public access to the data must be agreed by all companies.

Support the functions in preparing and compiling the dossiers and coordinating the work.
How many years does it take from discovering a new compound to being able to sell a developed and authorized product?
In 2019, the average time was 12.3 years. The increase of the required time is to a large extent driven by increasing requirements with regard to safety studies and risk assessments.
Crop Protection AI Discovery and Development Lead Time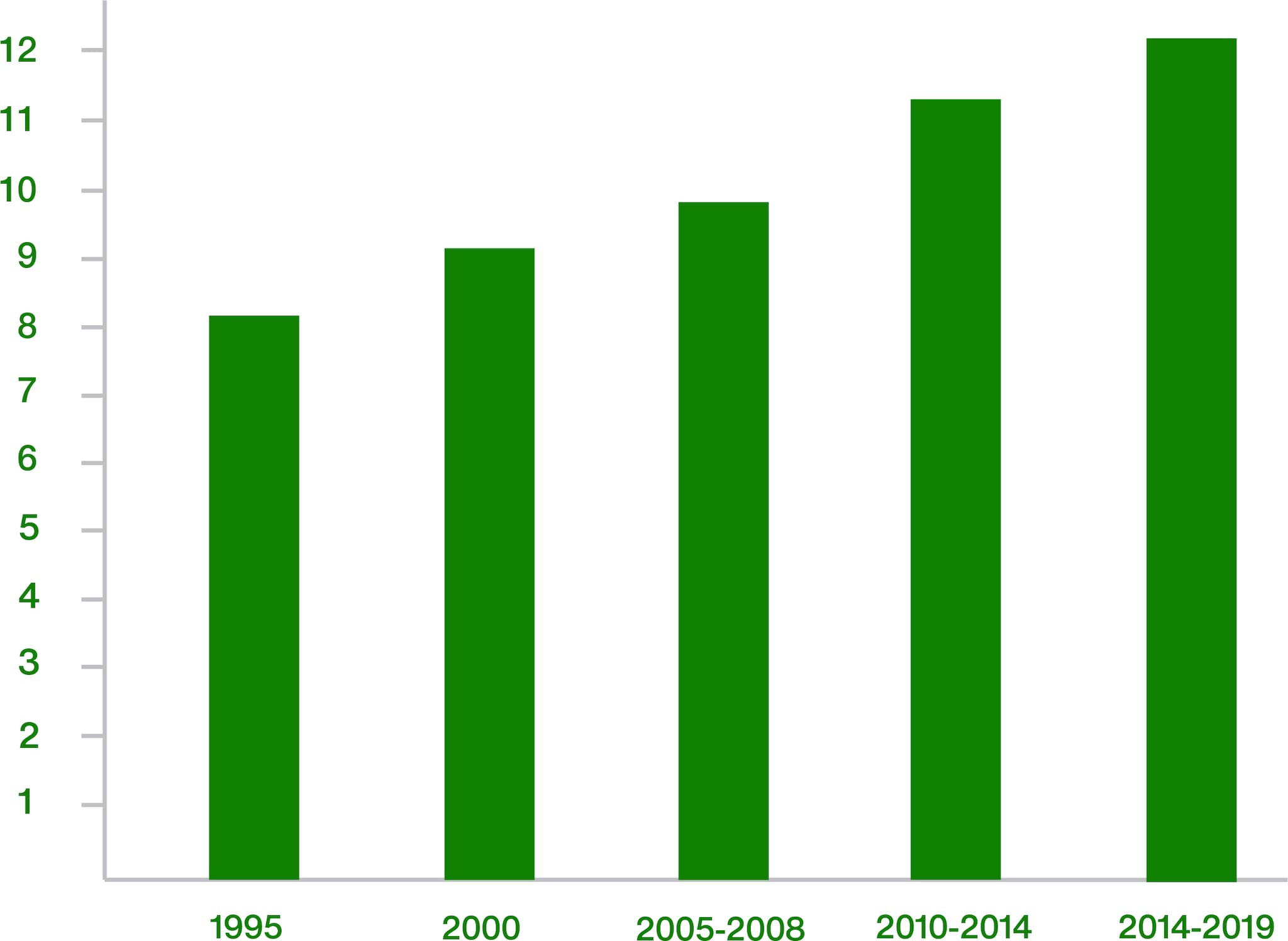
Number of years between the first synthesis and the first sale of the product containing AI.
*Source: AgbioInvestor (2024)
Did you know?
The number of molecules that had to be investigated to find one fit for developing into a product for market has increased from 52,500 in 1995 to 159,574 in 2014. This increase is mainly driven by stricter safety criteria.
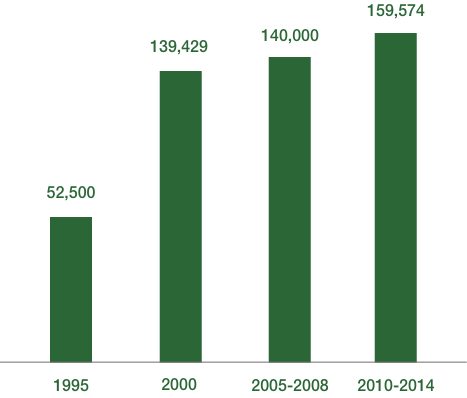
Number of molecules screened leading to a successful product launch.
Did you know?
Due to the high development cost and regulatory requirements, the number of substances developed has decreased from an average of 4 per year in 1995 to an average of 1.5 in 2014.
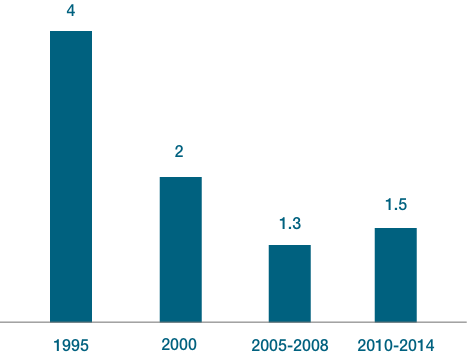
Number of substances developed per year leading to a successful product launch.
What to protect?
To decide whether a crop protection product is safe when used according to the label, we look at several areas.
Dietary Safety
Dietary safety evaluates the exposure to the human population, including sensitive populations such as infants and children, from potential residues of chemicals in food and drinking water to ensure the levels are safe.
Toxicology
Toxicology studies the adverse effects of chemicals in mammals to assess potential effects in humans.
Environmental fate
Environmental fate investigates the behavior, metabolism and distribution of substances in the environment (soil, water, groundwater, air).
Environmental Effects
Environmental effects assess the potential effects of chemical or biological agents to non-target organisms in the environment.
Occupational exposure
Occupational exposure evaluates exposure to workers resulting from the manufacture and use of chemicals and determines required personal protection equipment to ensure safety.
Product chemistry
Product chemistry determines the physical chemical properties of a chemical (e.g. solubility, volatility, chemical reactivity) that are important to ensure safe storage, transport, use and disposal of a chemical.
Agronomic development
Agronomic development evaluates how a product should be used in the field to ensure maximum efficiency and crop safety.
About Animal Testing
Animal testing is required under many regulations worldwide to ensure that pesticides and other chemicals (e.g. pharmaceuticals) are safe for humans with no unacceptable risks for the environment when used according to the label.
But everyone should know
Bayer works hard to minimize animal testing with intelligent testing strategies including in-vitro studies, modeling, avoiding double studies and sharing studies with other stakeholders.
- The regulatory process in the EU (Bayer)
- Video: The Regulatory Process to bring Pesticides to the Market (English, German, French, Spanish)
- Registering plant protection products in the EU (CropLife Europe)
- Authorization of Plant Protection Products (European Commission)
- How can we trust pesticide safety data? (Crop Life International)
Resource Library











