A developmental neurotoxicity screening study with technical grade Fenamidone in Wistar rats
BAYER CROPSCIENCE LP
Document Number: M-253958-01-1
Report Number: 201299
Date: 2005-06-29
Abstract
Technical-grade fenamidone was administered via the diet from gestation day (GD) 6 through lactation day (LD) 21 to mated female Wistar rats at nominal concentrations of 0, 60, 250, 1000 and 4700 ppm during gestation, with adjustments during lactation to achieve a more consistent dosage (mg/kg/day) throughout exposure. On postnatal day (PND) 4, litters with a minimum of eight pups, including at least three per sex, were culled to yield, as closely as possible, four males and four females. Subsets of surviving offspring, representing at least 20 litters per level, were subjected to the following observations and measurements - a functional observational battery (FOB), preputial separation or vaginal patency, body weight, food consumption, automated measures of activity (figure-eight maze), acoustic startle habituation, tests of learning and memory (passive avoidance after weaning and a water maze task on PND 60) and an ophthalmic examination. Neural tissues were collected from 10 rats/sex/dietary level (representing approximately 20 litters) on PND 21 (brain only) and at study termination (approximately 75 days of age) for microscopic examination and morphometry.
General
Based on analytical results, the mean concentrations of fenamidone in the diet during gestation were 0, 63.2, 271, 1045 and 4967 ppm for nominal concentrations of 0, 60, 250, 1000 and 4700 ppm, respectively.
The mean concentrations of fenamidone in the diet during lactation were adjusted to achieve a more consistent dosage (mg/kg/day), since food consumption increases during this time period. Levels of exposure (mg/kg/day) during gestation and lactation were as follows:
A.I. Intake (mg consumed/kg body weight/day)1

1 Dietary concentrations were reduced during weeks 1-3 of lactation (by factors of 1.9, 2.3 and 2.8, respectively), based on estimated increases in feed consumption (g consumed/kg body wt/day) during lactation).
2 Associated with observed food spillage and considered an unreliable measure of a.i. intake. This value was excluded from the mean average daily intake.
To avoid confusion between the text and tables, dietary levels are referred to throughout this report on the basis of nominal concentration during gestation.
The average daily intake of the active ingredient at nominal concentrations of 0, 60, 250, 1000 and 4700 ppm during gestation and with adjustments during lactation, was 0, 5.5, 23.2, 92.3 and 4293 mg/kg/day.
Reproduction parameters were not affected by treatment at any dietary level.
__________________________
3 Average daily intake calculation for high-dose dams excludes value from the first week of measurement due to food spillage.
Maternal
There were no compound-related deaths at any time or compound-related clinical signs during gestation or lactation at any dietary level.
During gestation, maternal body weight was not affected on any day at any dietary level, but weight gain (GD 0 to 20) was significantly reduced by 8% in high-dose animals. During lactation, maternal body weight was a maximum 5% (on LD 14) less than control in high-dose animals, but this difference from control was not statistically significant.
Food consumption was not affected by treatment at any dietary level during gestation or lactation.
Functional Observational Battery (FOB). During gestation and lactation, there were no compound-related findings at any dietary level.
Offspring (F1 Generation)
Mortality. There were no deaths ascribed to treatment at any dietary level.
Detailed observations. No compound-related signs were apparent at any dietary level.
Litter parameters. Compound-related effects were limited to decreased body weight and weight gain, which are described below.
Developmental landmarks (sexual maturation). No compound-related effects on preputial separation or vaginal patency at any dietary level.
Pupil constriction was not affected by treatment at any dietary level.
Body weight and weight gain. At birth, there was no difference in body weight at any dietary level. However, body weight was significantly reduced thereafter in high-dose males and females, such that they weighed significantly less than controls on PND 4 (9%) and on days 11, 17 (10%, each) and 21 (9%). In addition, weight gain for high-dose pups from PND 0 to 21 was significantly less than controls (9%). After weaning (when exposure was discontinued), body weight remained significantly reduced in high-dose males (3-5%) and females (4-6%) for the first three weeks post-treatment with complete recovery thereafter. Body weight and weight gain were not affected by treatment at lower dietary levels.
FOB. No compound-related effects were evident at any dietary level.
Motor and locomotor activity were not affected at any dietary level.
Acoustic startle habituation. No compound-related effects were evident at any dietary level.
Passive avoidance. No compound-related effects were evident at any dietary level.
Water maze. No compound-related effects were evident at any dietary level.
Ophthalmology. No compound-related lesions were evident at any dietary level.
Gross lesions. No compound-related lesions were evident at any dietary level.
Terminal body weight. Terminal body weight was not affected by treatment at any dietary level at PND 21 or study termination.
Brain weight. No compound-related effects were evident at any dietary level, on PND 21 or at study termination.
Brain morphometry. There were no differences in gross or microscopic brain measurements on PND 21 or at study termination at any dietary level.
Micropathology. There were no compound-related microscopic lesions on PND 21 (brain) or at study termination (brain, other neural tissues, and skeletal muscle).
Bayer CropScience (BCS) Response to EPA's Rejection of the Fenamidone Developmental Neurotoxicity Study (PC Code: 046679; DP Barcode: D319665)
BAYER CROPSCIENCE LP
Document Number: M-277523-01-1
Report Number: 201589
Date: 2006-09-12
In 2006, EPA provided BCS with a Data Evaluation Record (DER) on a DNT study (MRID 46590001) for the active ingredient fenamidone. Based on their review, EPA classified the DNT as "Unacceptable/Guideline and does not satisfy the guideline requirement for a developmental neurotoxicty study in rats (OPPTS 870.6300, § 83-6)." While several study deficiencies were noted, the primary reason given by EPA for finding the study unacceptable was as follows:
"Previously, the Health Effects Division suggested that a preliminary dose-range finding study be conducted if the Crl: CD (SD) BR strain of rat were not utilized in the required DNT, since the 2-generation reproduction study (MRID 45400014) was conducted with the Crl: CD (SD) BR strain of rat (Memo, PV Shah, 12-Jan-2004, TXR# 0052296). However, the DNT study was conducted with the Wistar Hannover strain of rat. It is therefore not possible to assess whether the decreases in absolute brain weight observed in the F1 and F2 generations in the 2-generation reproduction study using the Crl:CD(SD)BR strain of rat would also be observed in the required DNT paradigm, using the Crl:CD(SD)BR strain of rat."
In the following paragraphs, BCS provides a rebuttal to the Agency's rationale for rejecting the DNT study. Additional information in response to the study deficiencies noted in the DER is provided in Appendix 1.
Issue: Lack of reproducibility for brain weight findings between the studies.
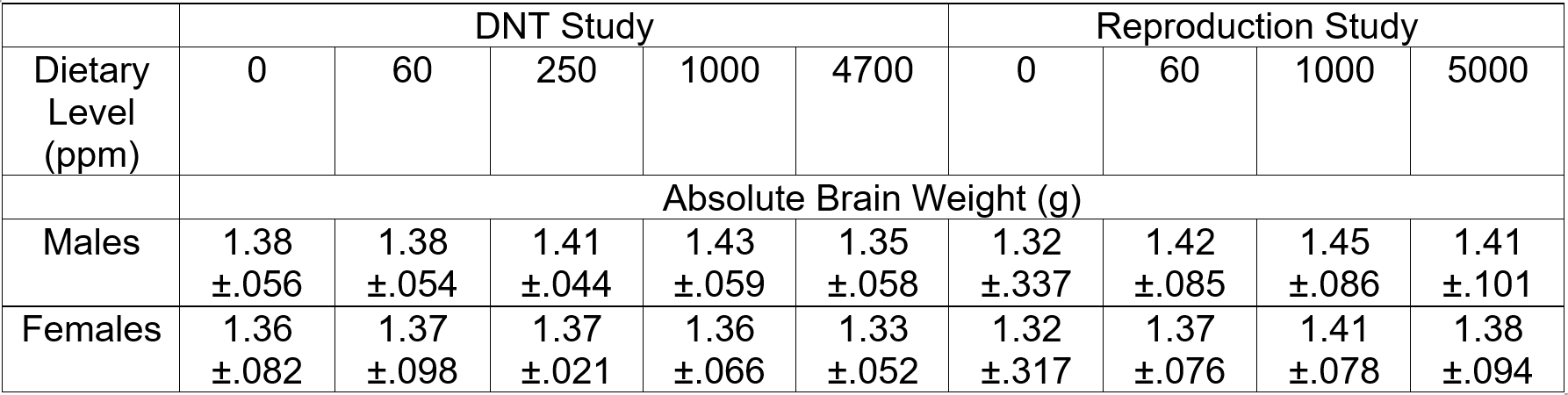
The Agency expressed concern that the brain weight differences reported in the 2-generation reproduction study were not replicated in the DNT study. Specifically, in the reproduction study, statistically significant decreases in absolute brain weight were observed in F1 female adults and F2 female pups in the mid- and high-dose groups (1000 and 5000 ppm, respectively) while no effect on brain weight was noted in F1 pups from any treatment group. Due to differences in study design, the only point for comparison that can be made is for the F1 pups in the DNT and the reproduction study. In a DNT study, F2 pups are not produced and exposure to F1 animals, allowed to reach maturity, is terminated at weaning on lactation day 21 (or postnatal day (PND) 21).
For the F1 pups at PND 21, the absolute brain weights were not affected by treatment in either the reproduction or the DNT study (Table 1) while pup weights at the high dose level were approximately 10% lower than control throughout most of the lactation period in both studies. Thus, the findings in the F1 pups, the only comparable set of animals between the two studies, are consistent across both of these studies despite the use of different rat strains.
Table 1: Comparison of Fl Pup Brain Weights (mean ± SD) in the DNT Study and 2-Generation Reproduction Study on PND 21
Issue: Acceptability of fenamidone DNT study.
When being evaluated for compliance to OPPTS Test Guidelines, each study should stand on its own merits regardless of the reproducibility of a specific effect or the strain tested. BCS believes that the DNT study was performed in full compliance with the US EPA (OPPTS 870.6300) guideline requirements, and the additional information included in this response to address study deficiencies should be sufficient to allow EPA to upgrade the study to acceptable.
In their review of the DNT study, EPA pointed out that they had previously suggested performing a dose range-finding study if the DNT was to be conducted with a rat strain different from the one used in the reproduction study. However, based on the extensive toxicological database available with fenamidone (including 90-day neurotoxicity, 2-generation reproduction, and long-term studies) and experience working with both rat strains, BCS considered the performance of a dose range-finding study to be unnecessary and indefensible from the perspective of responsible animal use. The dietary levels chosen for the DNT study (Wistar rat; 0, 60, 250, 1000, and 4700 ppm) were similar to those used in the 2-generation study (SD rat, 0, 60, 1000, 5000 ppm), the 90-day neurotoxicity study (SD rat; 0, 150, 1000, and 5000 ppm), and the long-term rat study (SD rat, 0, 60, 150, 1000, and 5000 ppm). Higher dietary concentrations of fenamidone have been associated with palatability problems and excessive toxicity which would likely confound interpretation of the study results, including brain weight data. In the long-term rat study, animals receiving the dietary level of 8000 ppm were terminated on Day 20 due to significantly lower bodyweight gains and evidence of severe physical distress. Thus, based on findings at 5000 ppm in the other studies, a dietary level of 4700 ppm was considered as an appropriate high dose level for a DNT study.
One issue that has long complicated the interpretation of reproduction data is that dietary intake and total compound exposure can almost double during lactation when the dam's demand for caloric intake significantly increases. In addition, pups can be exposed to excessively high doses of test material when they begin to consume diet at around PND 12. To counter this effect and retain a consistent mg/kg body weight dose for the dams throughout the DNT study, a decision was made to adjust the dietary concentrations during the lactation phase of this study. As the initial parental (F0) females in the high dose group of the reproduction study were exposed to approximately 460 mg/kg body weight/day during the pre-mating phase, a constant dose level of 400 mg/kg body weight/day was targeted as the high dose level in the DNT study. Despite adjustment of the dietary concentrations during lactation, body weight and weight gain for high dose pups from PND 4 through 21 was approximately 10% lower than control and was statistically different at most intervals. As a 10% decrement in body weight gain has been traditionally defined by EPA as sufficient evidence for achieving a Maximum Tolerated Dose (MTD), the highest dose tested in the fenamidone DNT study approximated an MTD for neonatal pups. All of the evidence indicates that higher dose levels would have further compromised pup weight and confounded interpretation of the neurological assessments due to lower weight pups. The DNT study is intended to evaluate the inherent potential of a compound to cause developmental neurotoxicity and should not be an assessment of delayed development associated with significantly lower weight pups.
For regulatory toxicity studies, neither the SD nor Wistar strain is considered to be more suitable for testing or more predictive of potential effects in humans than the other strain. The principal reason BCS chose to use the Wistar rat was because all DNT studies performed at BCS have used only this rat strain. Neurotoxicity endpoints have been validated using the Wistar rat and the availability of extensive historical control data in this strain aids in the interpretation of study results and identification of potential compound-related effects. Using a strain other than Wistar was not a feasible option in the absence of validation studies and historical control data for other strains which might have been utilized.
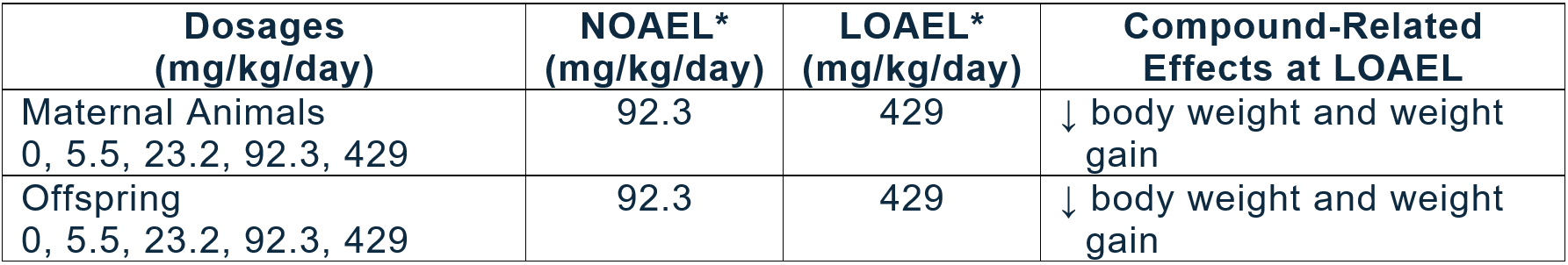
Issue - The maternal NOAEL is 4700 ppm (429 mg/kg/day)
BCS does not agree with the Agency conclusion that the maternal NOAEL is the highest dietary level (4700 ppm). In the DNT study at this dietary level, maternal body weight gain during gestation days (GD) 0-20 was statistically decreased by 8%, compared to control, which was largely due to a 27% decrease in body weight gain observed from GD 6-13. Such a large decrease in body weight gain is evidence of a treatment-related effect in the dams and thus, 4700 ppm cannot be considered as an NOAEL. Therefore, BCS reaffirms that the maternal NOAEL is 1000 ppm (92.3 mg/kg/day) and 4700 ppm (429 mg/kg/day) is a LOAEL, as stated in the original DNT report.
Conclusion
With the additional information included in this submission to address study deficiencies, BCS believes the Agency should now find the DNT study to be acceptable. Effects on body weight gain in dams and pups were observed and support the adequacy of 4700 ppm as the highest dose. Testing at a substantially higher level cannot be justified from an animal welfare perspective based on the humane sacrifice of animals in the long-term toxicity study after less than three weeks of treatment at 8000 ppm. Indeed, the bodyweight results in the DNT study are consistent with other studies in the fenamidone database and support the decision not to perform a dose range-finding study.
Regardless of any potential effects on brain at high dose levels, a clear NOAEL of 92 mg/kg/day was established in both the dams and pups in the DNT study. This dose level is slightly below the NOAEL of 125 mg/kg/day used by EPA to establish the acute RfD and significantly above the NOAEL of 2.83 mg/kg/day used for deriving the chronic RfD. Testing at higher dietary levels in an attempt to produce a lower absolute pup brain weight would have no impact on the existing NOAEL from the DNT study and would not impact risk assessment. Further, the weight of the evidence in the fenamidone database demonstrates similar levels of sensitivity in adult and young animals. Therefore, considering the completion of an acceptable DNT study, BCS believes that removal of the 10X database uncertainty factor is justified.
In case you would like to have access to the full study reports, please get in touch with us by filling out this request form and by accepting our Terms and Conditions for Access to Crop Protection Active Substance, Crop Protection Products and GM seeds and traits Study Documents.
Enter “M-253958-01-1” and “M-277523-01-1” in the study report identification field, list your name and email address on the order form and submit your request. Make sure that the email address you entered is valid. You will be sent an automatic reply by email, confirming that your request has been received.
The study report will be sent to you if all disclosure criteria are fulfilled (including the acceptance of our terms and conditions by you). Please note that, if this is the first time that a study report has been requested, delivery may be delayed. This is because the first time a report is shared, all personal data have to be redacted to protect the privacy of individuals in compliance with the General Data Protection Regulation (GDPR) and to minimize potential misuse of the report in a regulatory context.
To learn more, visit the Frequently Asked Questions page.








