Working with HCPs & HCOs
Bayer is committed to fostering transparency in its financial relations with healthcare professionals and healthcare organisations and believes that it creates trust in the innovative pharmaceutical industry.

|
|
Collaborative Research Benefits Patients and Society The collaboration between the pharmaceutical industry and both healthcare professionals and healthcare organisations has delivered numerous innovative medicines and changed the way many diseases impact our lives. We at Bayer believe this collaboration is key to achieving better outcomes for the patients we strive to help. |
|
Pharmaceutical Industry – Healthcare Professionals: A Highly Regulated Relationship When collaborating with medical experts, we comply with existing laws and regulations that clearly outline the interaction between industry and healthcare professionals, e.g. healthcare laws and industry codes. Additionally, these rules are amended by different transparency regulations, such as the Sunshine Act in the US, the European Federation of Pharmaceutical Industries and Associations (EFPIA) Disclosure Code (http://transparency.efpia.eu/ )in Europe, and more locally the Irish Pharmaceutical Healthcare Association (IPHA) Disclosure Code (http://www.ipha.ie/alist/publications.aspx?article=f8f9c1e9-344a-468c-b7d2-dc83530513b1). We fully respect the independence and integrity of these professionals. |
|
|
What the EFPIA Disclosure Code is About (http://transparency.efpia.eu/)
As a member of EFPIA and in line with our company values we fully endorse the EFPIA Disclosure Code. In Ireland, the implementation of this Code is carried out in accordance with the IPHA Disclosure Code. In Ireland, Bayer will disclose payments as well as benefits in kind to healthcare professionals and healthcare organisations on an online platform managed by IPHA. |
Industry and healthcare professionals collaborate in a range of activities in preclinical research, clinical development as well as clinical practice.
As the primary point of contact with patients, healthcare professionals offer invaluable and expert knowledge on patient experiences and the management of diseases. This plays a crucial role in optimising our collaborative efforts to improve patient care.
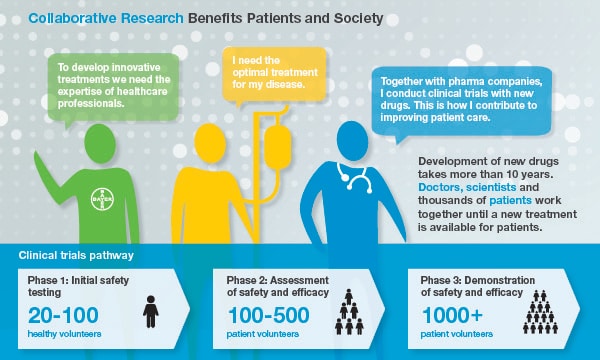
Collaboration example 1: Clinical Trials
Patients Need the Right Medicines
Patients can only truly benefit from the huge amount of invaluable medical knowledge that exists worldwide when our internal experts work closely together with external researchers and healthcare professionals. We strongly believe that the joint use of this expertise will help us to gain a better understanding of major diseases and to develop better medicines faster. The treating physician and other healthcare professionals conduct clinical trials at study centres in accordance with the clinical study protocol as approved by health authorities, institutional review boards, and ethics committees. Thus, they are the major link to the participating patients and are responsible for collecting data. Expertise and time offered by healthcare professionals and healthcare organisations in the course of a clinical trial need to be compensated [Link to What the EFPIA Disclosure Code is About/Level of Compensations].

Collaboration Example 2: Supporting Physician’s Education
Patients Need the Best Informed Doctors
Bayer supports the education of healthcare professionals at congresses and trainings. This contributes to ensuring doctors have access to the latest medical research. They are thus able to ensure that any treatment decisions made with their patients are based on the most up to date information available. Scientific events are just one of many resources for physician’s education.
Bayer fully supports greater transparency in the relationship between the pharmaceutical industry and healthcare professionals and organisations and therefore ensures compliance with the EFPIA Disclosure Code.
|
40 Leading European Companies in 33 Countries are Committed to the EFPIA Disclosure Code The EFPIA Disclosure Code (full title: EFPIA Disclosure Code of Transfers of Value from Pharmaceutical Companies to Healthcare Professionals and Healthcare Organisations) is a voluntary commitment that requires all EFPIA member companies to disclose transfers of value (“TOVs”) to healthcare professionals (“HCPs”) and healthcare organisations (“HCOs”). Under this code, all EFPIA member companies, including Bayer, will publish all direct and indirect, monetary and non-monetary transfers of value related to the development and commercialisation of prescription-only human pharmaceuticals. |
|
|
Level of Compensation Healthcare professionals are compensated for their expertise and the services they provide to the pharmaceutical industry. The level of payments and transfers of value depend on the kind of activity, level of expertise, and amount of time. The permissible amounts depend on various factors, e.g. local income level, governing laws and existing codes. The core principle is fair market value remuneration for services received to ensure that honoraria are not misused to unduly influence healthcare professionals in their treatment decisions. |
|
|
Transfers of value Transfers of value in the area of ‘Research and Development’ will always be reported on an aggregated level as regulated by the EFPIA Disclosure Code. Transfers of value to healthcare organisations will be reported on a named healthcare organisation basis. Transfers of value to healthcare professionals will be reported as described below. |
|
|
Legitimate Interest Effective from 1 January 2023 Bayer will switch from relying on written consent from HCPs, to legitimate interests as the legal basis of processing and disclosing transfers of value data under the applicable data protection legislation. We are proud of the support we provide to the healthcare industry for ongoing medical education and other permitted activities to improve patient care, and as such, we believe using legitimate interest as our legal basis for disclosure reporting allows us to be fully transparent to the industry, to patients and to society when reporting TOVs. Legitimate interest balances the interest of the company, principally to enhance transparency and trust within the industry, with the individual’s interests, rights or freedoms. Under legitimate interest, the default is that transfers of value will automatically be disclosed on an individual, named basis rather than in anonymously in aggregate. |
|
|
Legitimate Interest: What happens if an HCP decides they do not want their data disclosed? Once Bayer is relying on legitimate interest (from 1 January 2023), HCPs no longer have the option to opt-out and automatically have data reported in aggregate (anonymously). Instead, HCPs have the right to object to their data being processed but this is not an absolute right to prevent the activity. There must be a compelling reason that overrides Bayer’s legitimate interests (transparency and trust in the industry). There are very few reasons that would be sufficient to override this interest, but Bayer will assess each objection that is submitted in accordance with the process set out below Objection Process:
Please find further details in the : |
|
|
Consent Disclosure of transfers of value (“TOVs”) relating to activities occurring before 1 January 2023 will be disclosed individually only with written consent from the healthcare professional. Should a healthcare professional not grant his/her consent for individual disclosure, then transfers of value to such a healthcare professional will be reported on an aggregated no-name basis. |
|
|
Data Privacy Bayer fully respects data privacy and data security. We therefore take multiple steps to protect data in compliance with the data privacy policies required and audited by local and global authorities. Bayer implements data security measures to ensure data are protected against external attacks and manipulation. In addition, access to any personal disclosure related data is restricted internally to employees responsible for data collection or report preparation. |
In accordance with the EFPIA Disclosure Code Bayer discloses transfers of value connected to the development and commercialisation of prescription-only human pharmaceuticals to healthcare professionals and healthcare organisations.
Please find further details in the Privacy statement for disclosures of transfers of value 2023 onwards- Ireland
The disclosed data include transfers of value in four main categories:
| Categories | Transfers of value | |
| Healthcare Professionals | Healthcare Organizations | |
| Donations and grants | - | Whether monetary or other |
| Events | 1. Registration fees 2. Travel & accommodation | 1. Sponsorship 2. Registration fees (if applicable) 3. Travel & accommodation |
| Services and consultancy | 2. Related expenses agreed in the contract (including, for example, travel, accommodation & registration fees) | |
| Research and development | Fees, travel & accommodation, and other related expenses that are covered in connection with the service | |
| Get Access to the Ireland Data > www.transferofvalue.ie |
Learn More About our Disclosure Methodology
The general rules of the EFPIA Disclosure Code apply to all member companies and all companies will disclose transfers of value made to healthcare professionals and healthcare organisations in a pre-defined format. However, some details of the reporting methodology are left for the individual companies to decide in order to allow the necessary flexibility to adjust to the internal processes. This methodology note allows you to understand how Bayer in Ireland is documenting and disclosing the relevant information.